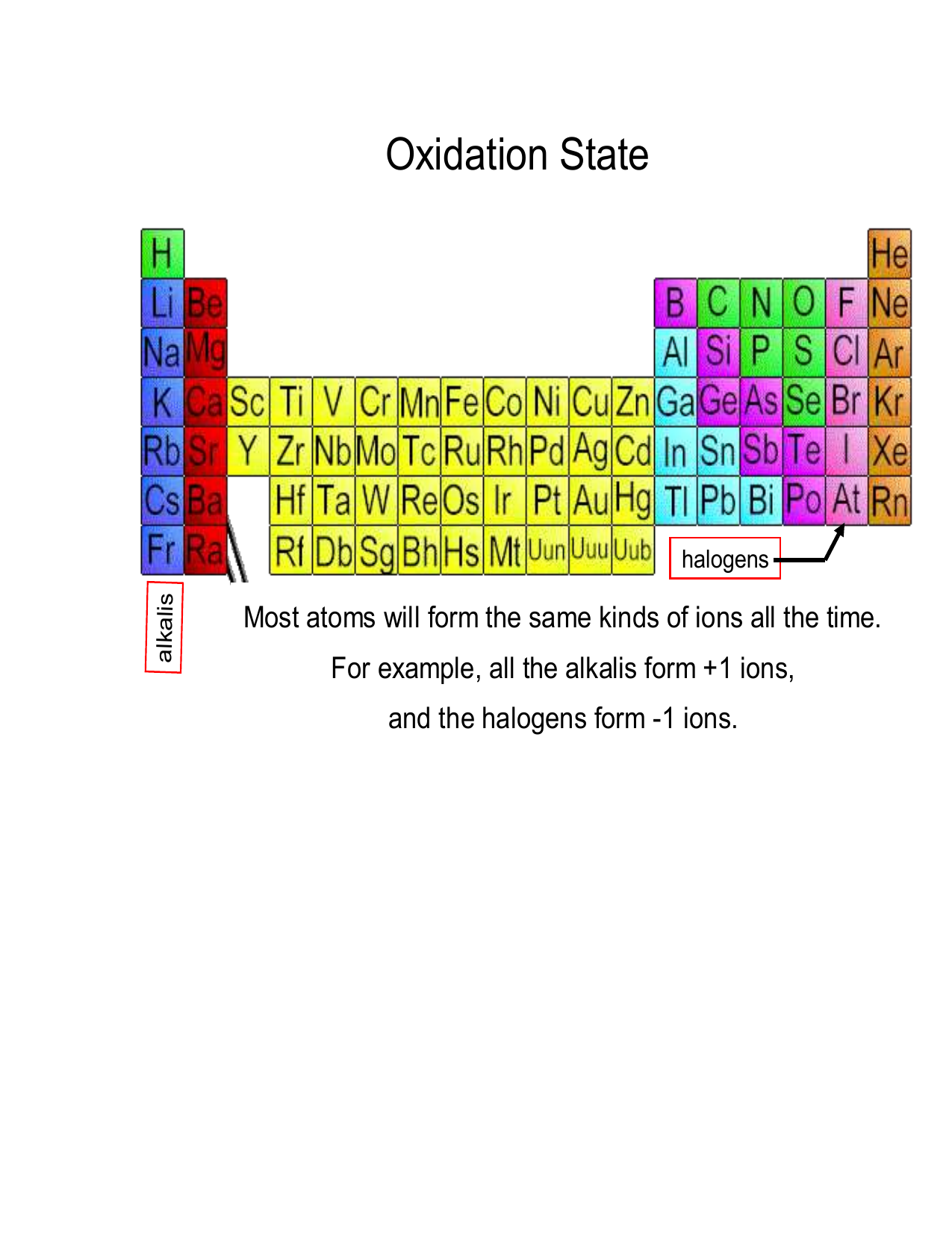
Introduction to Oxidation States
The concept of oxidation state is fundamental in the field of chemistry. It plays a crucial role in understanding chemical reactions, particularly redox reactions. The oxidation state, also known as oxidation number, is a theoretical charge that an atom would have if all bonds to atoms of different elements were completely ionic. This concept helps chemists keep track of electron transfer in chemical processes. In this guide, we aim to provide an in-depth understanding of oxidation states, their calculation, and their significance in various chemical contexts. Whether you're a student, a professional chemist, or just an enthusiast, grasping this concept is essential for appreciating the intricacies of chemical reactions.
The Basics of Oxidation States
Oxidation states are assigned to atoms based on a set of rules that make the process systematic and predictable. These rules consider the electronegativity of atoms, the type of bonds they form, and their position in a molecule. For instance, in a neutral molecule, the sum of the oxidation states of all atoms must equal zero, while in ions, it must equal the charge of the ion. The oxidation state of an atom in its elemental form is always zero. Understanding these basic principles is the first step toward mastering the concept of oxidation states. With these foundations, we can explore more complex molecules and reactions with confidence.
How to Determine Oxidation States
Rules and Guidelines
Determining the oxidation state of an atom in a compound involves following specific rules. Firstly, the oxidation state of a free element is zero. For a simple (monatomic) ion, the oxidation state is equal to the ion's charge. Oxygen typically has an oxidation state of -2, while hydrogen usually has +1. However, exceptions exist, such as in peroxides or when hydrogen is bonded to metals in hydrides. The sum of oxidation states in a neutral compound is zero, and in a polyatomic ion, it equals the ion's charge. These guidelines make it easier to assign oxidation states systematically, ensuring consistency across various chemical scenarios.
Significance of Oxidation States in Redox Reactions
Redox reactions, or oxidation-reduction reactions, are processes where electrons are transferred between substances. Understanding oxidation states is critical for identifying which species are oxidized and which are reduced. Oxidation involves an increase in oxidation state, while reduction involves a decrease. By analyzing the changes in oxidation states, chemists can determine the electron flow within reactions. This information is crucial for balancing redox equations and for applications such as electrochemistry and industrial processes like metallurgy. Mastery of oxidation states provides insight into the electron dynamics of chemical reactions, making it a vital tool for chemists.
Applications of Oxidation States in Real-World Chemistry
Oxidation states are not just theoretical constructs; they have practical applications in the real world. For instance, they are used in determining the feasibility of chemical reactions, especially in electrochemistry where they help calculate cell potentials. In environmental chemistry, oxidation states help in understanding the behavior of pollutants and the mechanisms of their degradation. In industrial chemistry, processes such as corrosion and combustion are analyzed through the lens of oxidation states to improve efficiency and prevent damage. Thus, oxidation states serve as a bridge between theoretical chemistry and practical applications, highlighting their importance beyond academic settings.
Common Misconceptions About Oxidation States
Despite their importance, oxidation states can be misunderstood, leading to common misconceptions. One such misconception is that oxidation states always represent real charges on atoms, whereas they are often formal charges used for bookkeeping in reactions. Another is the confusion between oxidation states and valency, which are related but distinct concepts. Understanding these differences is crucial for accurate chemical analysis and communication. By addressing these misconceptions, chemists can avoid errors in reaction predictions and ensure clarity in their work.
Oxidation States in Transition Metals
Transition metals present a unique challenge and opportunity in the study of oxidation states. These metals can exhibit a range of oxidation states due to their ability to lose different numbers of electrons from both the s and d orbitals. This variability allows transition metals to form a vast array of compounds with diverse chemical properties. Understanding the oxidation states of transition metals is key to exploring their roles in catalysis, coordination chemistry, and materials science. This complexity adds depth to the study of oxidation states, showcasing the versatility and adaptability of transition metals in chemistry.
Advanced Concepts: Fractional Oxidation States
In some cases, compounds exhibit fractional oxidation states, which can initially seem perplexing. These occur in compounds where electrons are delocalized, such as in certain metal oxides and mixed-valence compounds. Fractional oxidation states indicate the average electron distribution among identical atoms within a molecule. Understanding these states requires a deeper exploration of molecular orbitals and electron delocalization, offering a more nuanced view of electron distribution in complex compounds. This advanced understanding broadens the scope of oxidation states, pushing the boundaries of traditional chemical concepts.
Educational Strategies for Learning Oxidation States
For students and educators, mastering oxidation states can be facilitated through various educational strategies. Visual aids such as oxidation state charts and diagrams can simplify complex concepts, while hands-on laboratory experiments provide practical experience. Interactive simulations and online resources offer dynamic learning opportunities, allowing learners to explore oxidation states in virtual environments. These tools, combined with traditional teaching methods, can enhance comprehension and retention of oxidation state concepts, fostering a deeper understanding of their role in chemistry.
Conclusion: Embracing the Complexity of Oxidation States
The study of oxidation states is a journey through the intricacies of chemical reactions and electron transfer. From their basic principles to advanced applications, oxidation states offer valuable insights into the nature of chemical bonds and reactions. By embracing the complexity of oxidation states, chemists can unlock new possibilities in research, industry, and education. As we continue to explore the chemical world, the understanding of oxidation states will remain a cornerstone of chemical knowledge, guiding us to greater discoveries in the years to come.
You Might Also Like
Understanding Absolute Neutrophils: A Key Component Of Your Immune SystemExploring Iconic NYC: A Journey Through The Heart Of The Big Apple
Exploring The Westward Expansion: A Journey Through Time
Understanding Chronic Gastritis: Causes, Symptoms, And Management In 2024
Understanding Armpit Pain: Causes, Tips, And Remedies In 2024
Article Recommendations
- Unveiling Paula Wallace Net Worth A Journey Of Success And Wealth
- The Enduring Legacy And Remarkable Journey Of Terence Stamp 2024
- Lisa Henson Net Worth A Detailed Analysis Of Her Life And Financial Success